Is Bottled Spring Water a Compound Element or Mixture
Distilled water has also been purified in the process of distillation to temove any traces of impurities dissolved in it leaving just the wayer mllecules. Most things you use every day are a.

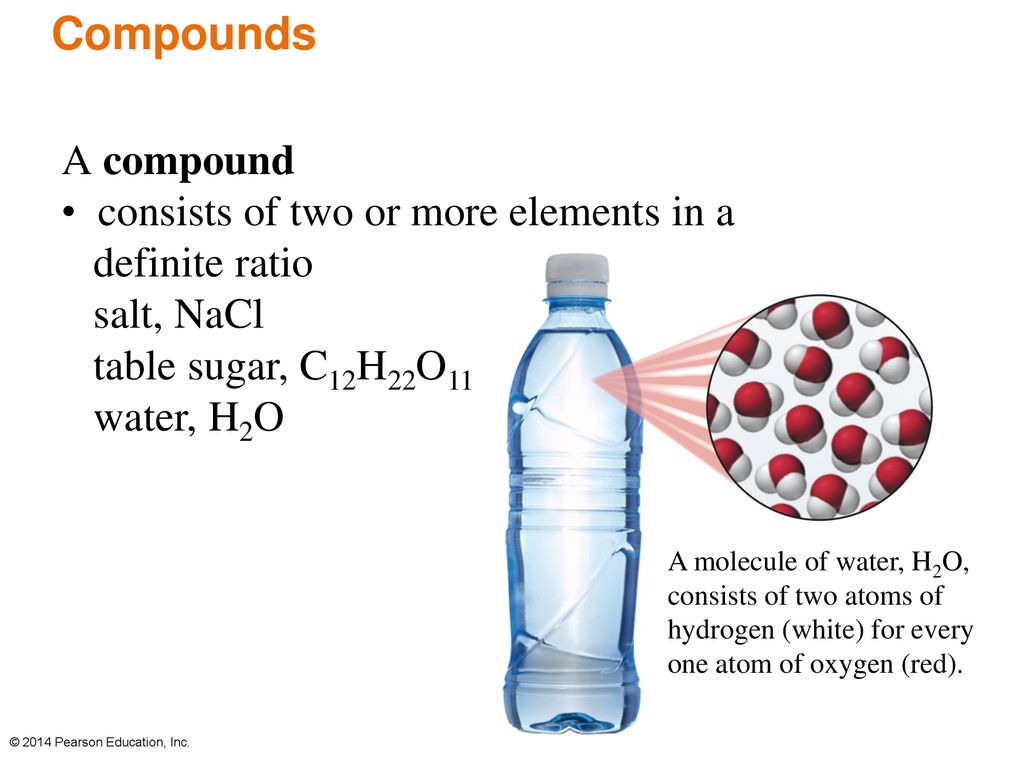
What Is A Solution Bottled Water Is A Solution Ppt Video Online Download
Therefore water is not a mixture.

. YOU MIGHT ALSO LIKE. Explain your reasoning for each. It is not a mixture because it only contains one type of molecule.
In a mixture the ratio of substances can ____. Bottled Spring Water f. See the answer See the answer See the answer done loading.
Water- hydrogen and oxygen atoms chemically bonded together. If it is a substance write element or compound in the appropriate column. Most of the time pure water has to be created.
By some form of chemical bond. A stainless steel fork. Explain the reason for each choice.
Identify each of the following as element compound homogeneous mixture or heterogeneous mixture. Distilled water is pure liquid H2O which makes it a compound. How are the compounds water and carbon formed.
Explain your answer a. Pure water is called distilled water or deionized water. Identify each of the following as an element compound homogeneous mixture or heterogeneous mixture.
Distilled water as long as its not contaminated with any other elements or compounds is a compound. Click card to see definition. As it is a pure water H2O its a compound.
Mixtures can occur between all phases of matter. Click card to see definition. By the process of distillation all the impurities are removed from the water.
Identify each of the following as element compound homogeneous mixture or heterogeneous mixture. Element Mixture Compound Sort. Element Compound Homogeneous Colloid Suspension Heterogeneous Solution Oxygen Water Alloy Milk Orange Juice Dirt.
A group of two or more atoms joined together by chemical bonds. Glass nad been used to called cellulose nit chemical had evap plastic film on the Initially Benedictus shatter-resistant gl manufacturers but interested. A pure substance that cannot be broken down into simpler substances by physical or chemical means.
Alloy bicycle frame i. A orange juice b aluminum foil c vegetable oil d baking soda This problem has been solved. Bottled spring water is a mixture mainly water with small amounts of dissolved salts.
Identify each as an element compound heterogeneous mixture or homogeneous mixture. Carbon- carbon and oxygen atoms combined together. In compounds 2 or more elements have been combined in a ____ ratio.
Ingredients keep individual properties. Different kinds of atoms that are bonded together. Water H2O is a pure substance a compound made of hydrogen and oxygen.
Is water an ionic molecule. Neither the water nor the bottle are elements. A compound contains two or more elements bonded together ie.
Click again to see term. Water has the formula H2O so it is made of two elements bonded together. Give an example for each of the following mixtures.
Although water is the most abundant substance on earth it is rarely found naturally in its pure form. It is also not an element because water molecules are made of both hydrogen and oxygen. Classify each of the following as either substance or mixture.
Tap again to see term. Distillation is bolling of water condensing the steam into liquid form of pure water. Is substance that contains two or more different elements chemically joined in that has the same composition throughout.
It is a compound and it is pure. What is a compound. Is water is a mixture.
Tap card to see definition. Pure water is a compound but most bottled waters are mixtures as they contain traces of minerals. Is bottled spring water a compound element or mixture.
Tap water and bottled water spring water are mixtures containing dissolved minerals calcium carbonate and magnesium sulfate. Tap card to see definition. Element - It is pure substance made up of same atoms having same no.

Everything You Need To Know About Bottled Water Labels

0 Response to "Is Bottled Spring Water a Compound Element or Mixture"
Post a Comment